Our Technology
IOP and outflow facility: role of trabecular meshwork (TM)
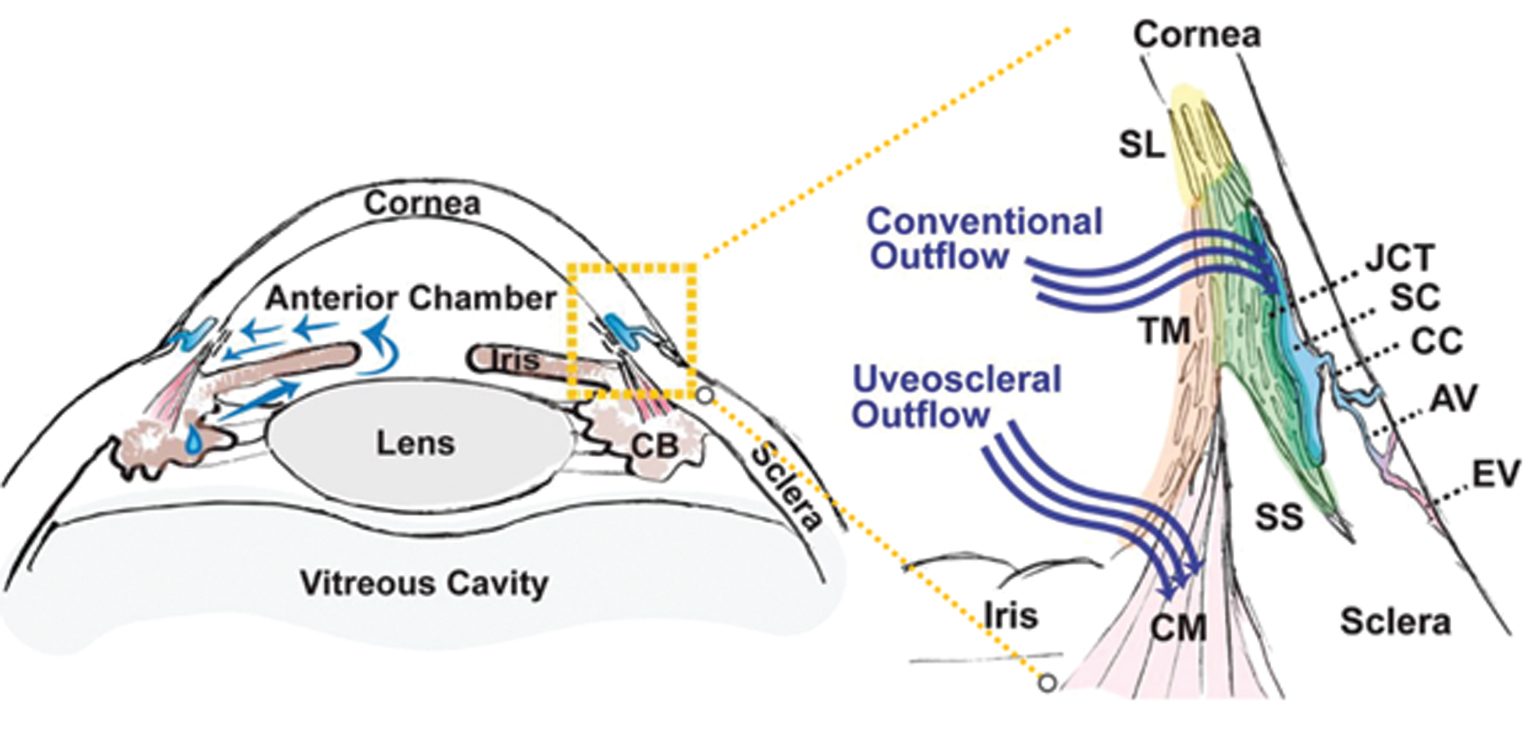
Intraocular pressure (IOP) is determined by the balance between constant aqueous humor production and its elimination from the eye into the systemic circulation. Open angle glaucoma (OAG) is caused by a reduction in aqueous elimination through the outflow tissues.
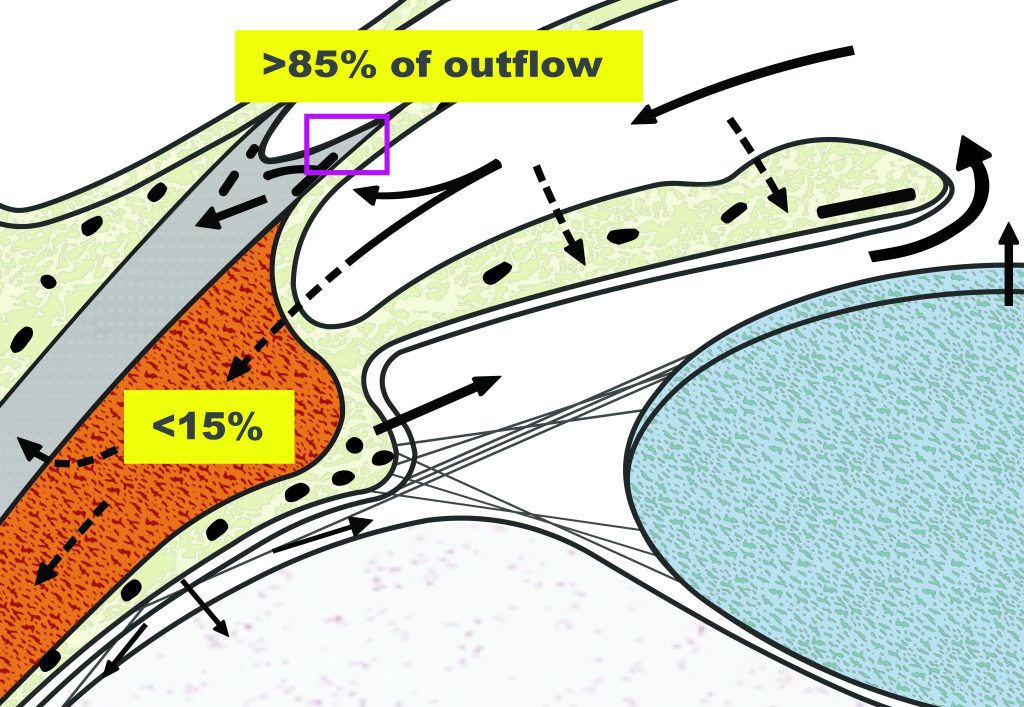
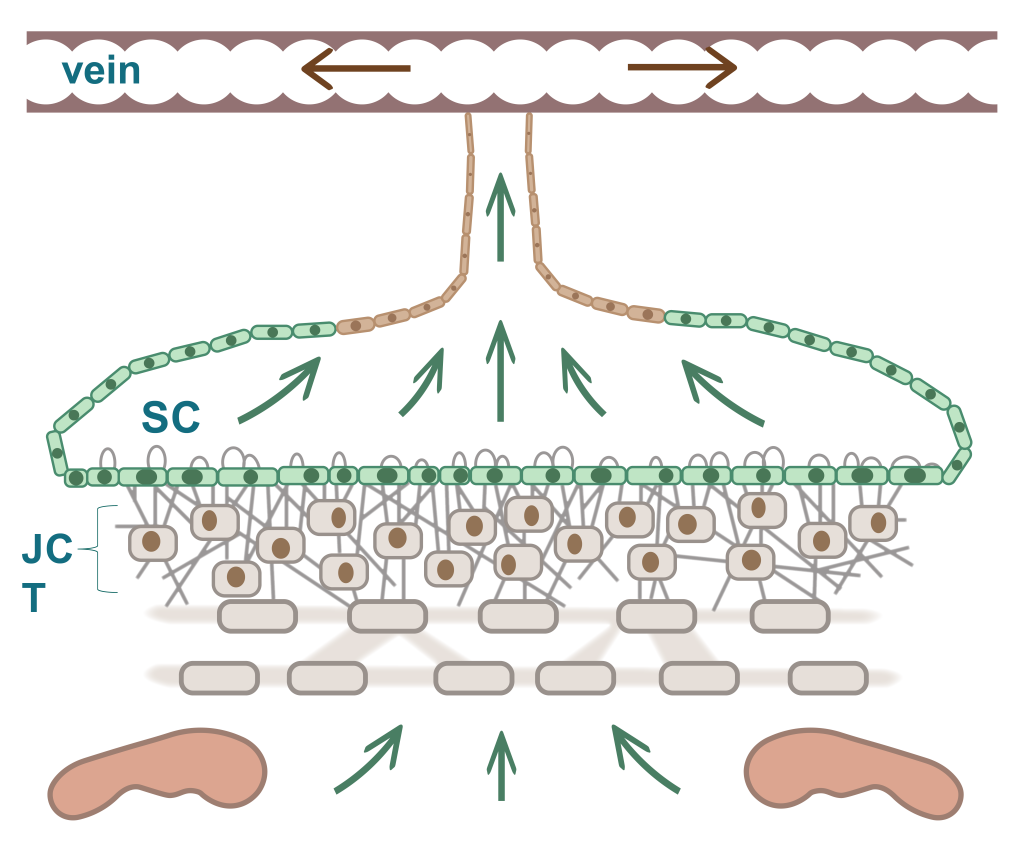
The trabecular meshwork (TM) is a three-dimensionally arranged heterogeneous tissue located in the anterior chamber angle and is composed of collagenous beams populated with TM cells. Together with the adjacent inner wall of Schlemm’s canal these “outflow tissues” handle about 85% of the aqueous outflow. These tissues are the main point of resistance to aqueous outflow (the highest resistance is in the juxtacanalicular tissue, JCT) and thus largely control IOP.
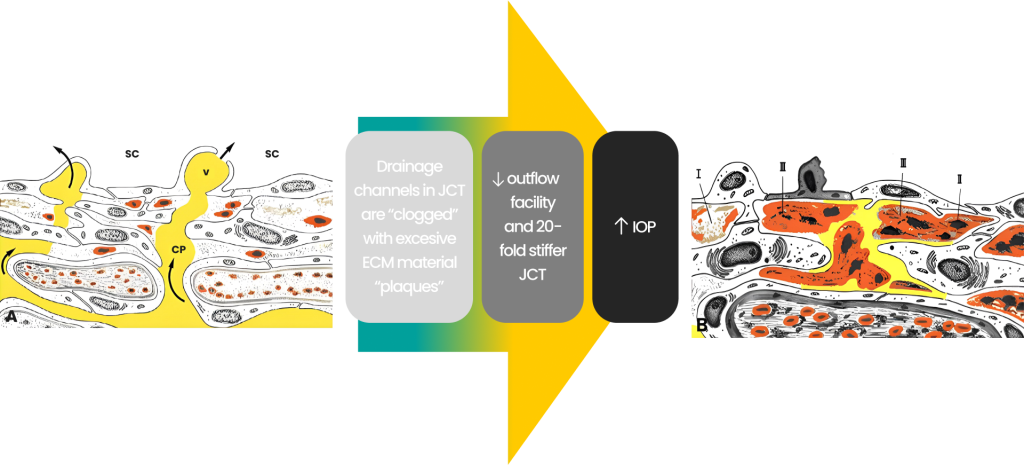
Glaucoma: excessive extracellular matrix (ECM) decreases outflow
Malfunction or pathology of the outflow tissues leads to increased outflow resistance and IOP elevation. The drainage channels in the JCT become “clogged” with excessive extracellular matric (ECM) material “plaques”. The ECT becomes 20-fold stiffer than normal, decreasing the outflow facility resulting in high IOP and glaucoma.
3.1 IOP and TM/outflow facility
Glaucoma: excessive extracellular matrix (ECM) decreases outflow
Normal
trabecular
meshwork
Glaucomatous TM
Mode of Action and tPA
We have shown that tPA supplementation or upregulation can reverse steroid-induced outflow facility reductions in mice and IOP elevation in sheep, and suggested that matrix metalloproteinases (MMPs) are at least partially mediating the tPA effect on outflow facility. MMPs are known to degrade and modulate the ECM in the TM and the adjacent outflow tissues, with a relatively straightforward MOA that links tPA to increased outflow of aqueous humor from the eye and reduction in IOP.

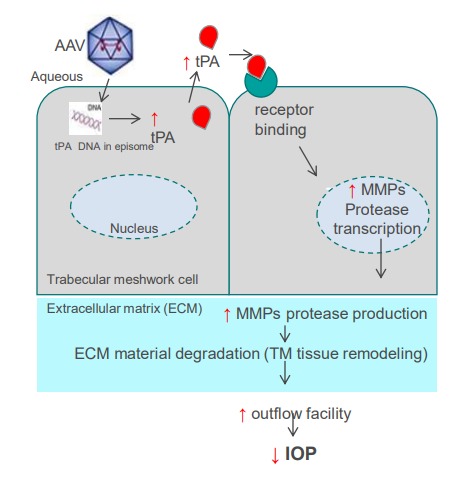
3.2 MoA and tPA

Gene therapy
The novelty of our approach is that we target gene-independent disease-modifying MOA therapy for open angle glaucoma (OAG) that will generate our engineered variant of tissue plasminogen activator (tPA) in the trabecular meshwork (TM) that drains aqueous fluid from the eye, resulting in long-term improved outflow facility and decreased IOP after a single intracameral injection.
We are using AAV vector to deliver engineered tPA. Our tPA upregulates multiple enzymes called metalloproteinases ( MMPs) which modulate the extracellular matrix composition in the TM and the adjacent outflow tissues leading to increased outflow of aqueous humor from the eye and reduction in IOP.
Our gene therapy is applicable to all OAG patients, independent of genetic background.
Gene therapy
The novelty of our approach is that we target gene-independent disease-modifying MOA therapy for open angle glaucoma (OAG) that will generate our engineered variant of tissue plasminogen activator (tPA) in the trabecular meshwork (TM) that drains aqueous fluid from the eye, resulting in long-term improved outflow facility and decreased IOP after a single intracameral injection.
We are using AAV vector to deliver engineered tPA. Our tPA upregulates multiple enzymes called metalloproteinases ( MMPs) which modulate the extracellular matrix composition in the TM and the adjacent outflow tissues leading to increased outflow of aqueous humor from the eye and reduction in IOP.
Our gene therapy is applicable to all OAG patients, independent of genetic background.

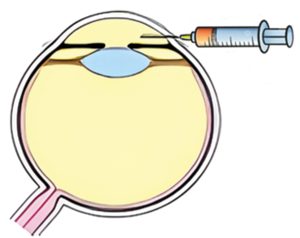
Intracameral delivery
A single in-office intracameral injection in the anterior chamber provides direct access to the TM cells (target cells) directly on the path of normal draining of the aqueous through the TM.
Ophthalmologists and especially glaucoma specialists are trained and familiar with intracameral injections, which are part of regular clinical practice.
